Monkeypox: ‘This is an entirely new spread of the disease’
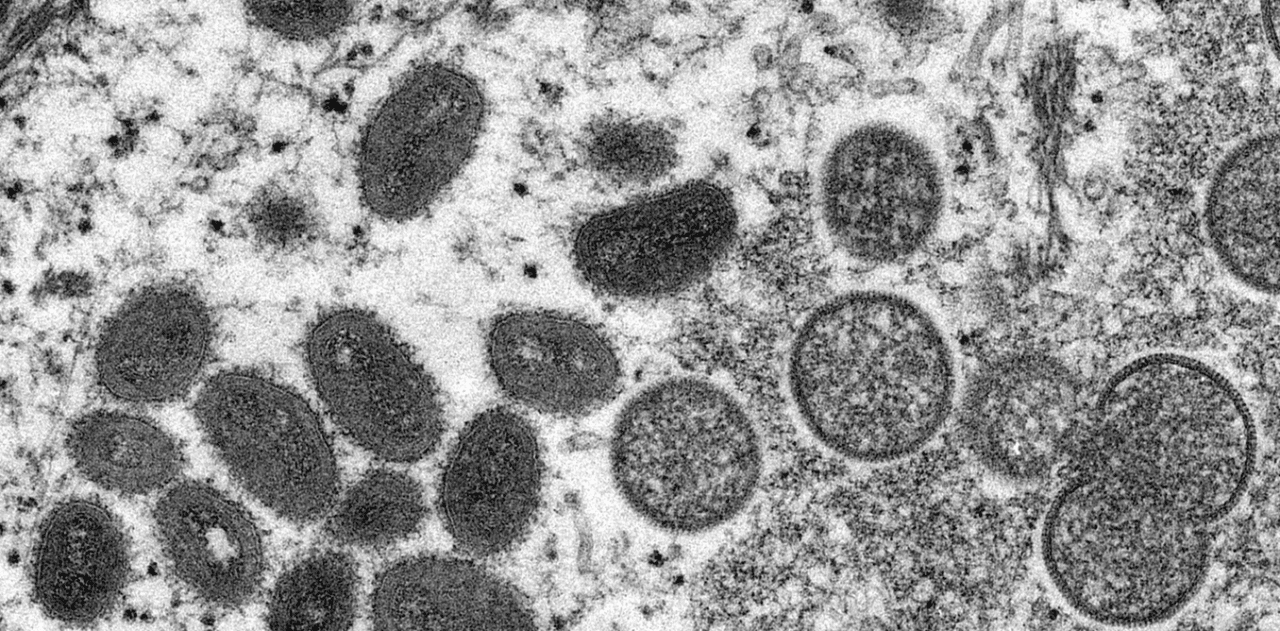
1,000 confirmed cases of monkeypox, a disease originating in Africa, have been recorded since early May across at least 30 non-endemic countries such as the United Kingdom, Spain, Portugal, France, the United States, Australia, United Arab Emirates and Israel. But what is this virus? Who is affected? And should we be worried about this recent surge in cases? In a bid to answer such questions, we caught up with Camille Besombes, a medical doctor specialist in infectious diseases, has been involved for the past three years in Afripox, a project that aims to gain a better understanding of the virus in its endemic region. She is currently conducting PhD research within the unit headed by the project’s coordinator, Arnaud Fontanet, a leading medical epidemiologist and emerging infectious disease specialist at the Pasteur Institute.
The Conversation: What exactly is the monkeypox virus?
Camille Besombes: Monkeypox is a virus belonging to the genus Orthopoxvirus, a family that also includes smallpox. Like smallpox, it is a large DNA virus with a particular appetite for skin tissue. However, smallpox only affected humans, which meant that we were able to eradicate it through worldwide mass vaccination, whereas monkeypox is carried by an animal viral reservoir. And despite its name, the natural reservoir is not actually monkeys.
The term “monkeypox” was coined when the virus was first identified in captive primates (in Denmark back in 1958), but in nature, the virus is most often found in squirrels and other rodents. In 1970, the first human case of monkeypox was documented in a nine-month-old child in the Democratic Republic of the Congo, amid increasing efforts in the campaign to eradicate smallpox.
There are two strains of monkeypox that we know of. The type that affects Nigeria, Liberia, Sierra Leone, and Côte d’Ivoire is the so-called “West African” strain, with a case-fatality rate between 1 to 3%. This is the one that was detected in the recent cases in Europe. The second is the “Congo Basin” strain, which circulates in the Democratic Republic of the Congo (DRC), the Republic of the Congo, the Central African Republic (CAR), and Gabon. Both strains are now circulating in Cameroon: recently, cases of infections implying the West African strain – imported from Nigeria – have been reported. Associated with more severe clinical forms, the Congo Basin strain has a case-fatality rate of around 10%.
We must also keep in mind that these figures are taken from countries where medical care is somewhat lacking, particularly in more remote regions. As for Europe, several patients are currently in hospital with the disease, but no death and no severe form of it have been detected on the continent.
TC: What are the symptoms of this disease?
CB: Following a relatively long incubation (usually lasting around 6 to 13 days, and up to 21 days), it presents its first onset symptoms during a two-day period known as the “prodromal” phase. These symptoms may include high fever, headaches, swollen lymph nodes (which are a sign that distinguishes it from smallpox), muscle pain, and fatigue. It is at this stage that patients are considered to be contagious.
Monkeypox causes lesions that gradually spread over the infected person’s body.
Jean-Marc Zokoé, Fourni par l’auteur
Next, the patient develops a rash, usually starting on the face and gradually spreading over the rest of the body. This rash causes pain and intense itching due to the inflammation that occurs around the skin lesions. In the West African strain, these lesions can be initially rather infrequent and discreet, and may therefore go unnoticed. The disease typically lasts two to four weeks and tends to go away spontaneously in the majority of cases.
The main complications of monkeypox include dehydration due to water loss from numerous and more widespread lesions, secondary bacterial infection of the lesions, sepsis, and corneal or other ocular lesions that may lead to vision loss. On top of these, cases of encephalitis (ed. note: “inflammation of the brain”) [have also been documented], most notably in a child during the 2003 US outbreak.
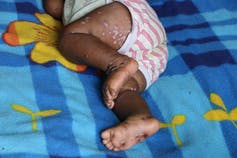
Children are more at risk of developing more severe forms of the disease.
Jean-Marc Zokoé, Fourni par l’auteur
Children who have been infected with monkeypox are more likely to experience complications and therefore have a higher fatality rate than adults. It is also assumed that immunocompromised individuals (particularly those who are HIV-positive) have a higher risk of developing a severe form of the disease, but there is not enough data yet to know this for certain. During the 2017-18 Nigerian outbreak, four out of seven people who died from the disease were HIV-positive. Pregnant women could also be affected by less moderate forms and we noted instances of mother-to-child transmission.
Treatment of the disease is largely symptom-based and involves methods like disinfecting the lesions, administering antibiotics in cases of secondary infection, and rehydration. Research is currently being conducted into whether certain antiviral molecules (such as tecovirimat could be effective against monkeypox, but the results are not yet conclusive.
TC: Is this the first time that the virus has spread outside of the African continent? How many cases have been recorded so far, and where?
CB: No, it isn’t the first time. Although the Congo Basin strain has never travelled beyond Africa, the West African strain managed to reach the United States in 2003 by way of imported animals that had been infected. More recently, however, a number of countries have reported several cases brought in by humans.
Back in 2003, a number of individuals in the US caught the virus from infected prairie dogs purchased from pet shops where the animals had been in contact with monkeypox-carrying Gambian pouched rats (Cricetomys gambianus) imported from Ghana. A total of 47 suspected cases of human infection were recorded, all the result of zoonotic (i.e. animal-to-human) transmission. There were no instances of interhuman transmission. At the time, the US authorities were concerned that the virus might take over a reservoir of local species, but this did not happen.

Tammy Kautzer tends to her prairie dog in Dorchester, Wisconsin, 9 June 2003. This mother was among a number of US residents that year required to quarantine in their homes after coming into contact with prairie dogs infected with the monkeypox virus.
Mike Roemer/Getty Images Nord America/AFP
Then, in September 2017, a more severe outbreak occurred in Nigeria, which had not experienced any monkeypox epidemic in the preceding 39 years. This particular epidemic is still ongoing, sustained by sporadic, regular transmissions that are both zoonotic and interhuman. To date, at least 500 suspected cases have been declared (215 of which have been confirmed). Reassuringly, albeit regrettably, only 8 deaths have been documented in the past 5 years.
However, the Nigerian epidemic had marked a major change in monkeypox epidemiology and should have acted as a warning to us. Whereas the virus had tended to thrive in forest regions with little connection, in 2017, it hit the country’s more urban areas and at a larger scale. This is how it managed to spread more easily beyond the continent, with cases popping up in 2018 in Singapore, Israel, and England, brought back by travellers returning from Nigeria.
In the case of England, a local human-to-human transmission occurred when a British healthcare worker became infected while cleaning a patient’s bed. There was no endemic viral circulation at the time, but more infections emerged in 2021, again linked to travellers coming back from Nigeria and occurring both in the UK and in the US (where two cases were recorded).
In the UK in 2018, scientists also studied the risk of emergence of an endemic animal reservoir. Species such as the common squirrel (Sciurus vulgaris) and domestic mouse (Mus musculus) were thought to be particularly prone to the virus, while rodents (vole, dormice, mice, hedgehog) were also considered as potential reservoirs.
TC: What is different about the current context?
CB: The situation is very different this time around. We know that the first case of the current epidemic, recorded on 7 May in the UK, was that of an individual travelling back from Nigeria. However, several other UK cases have since been confirmed that are apparently unrelated to one another or to this 7th May case. No instance of foreign travel (to African countries) associated with the infections has yet been proven and the direct chains of transmission have not been identified, suggesting the existence of several chains of transmission and a local circulation of the virus.
As of 6th June, 1,000 cases had been detected in at least 30 different countries, worldwide, with the largest number of cases located in the United Kingdom (287 confirmed), Spain (189 confirmed), Portugal (143 confirmed). French authorities have reported 51 confirmed cases. For now, all of the recorded infections outside of Africa have been mild. Only a few patients have been hospitalized and no death or vital threat have been reported.
That said, these local circulations of the disease are unprecedented. Another new aspect is that the cases have almost exclusively been reported among young males, primarily among homosexual men (in the UK, the authorities emphasized that “currently most cases have been in men who are gay, bisexual or have sex with men”). Only six suspected and confirmed women were declared in Spain, Czech Republic, Italy, USA and United Arab Emirates. These last two women were not linked to the European cluster following mass gathering events, but returned from Western Africa, suggesting something is up with the Nigerian epidemic that is exporting the virus.
TC: Why is this new? What are the usual channels of infection?
CB: Monkeypox epidemics most often arise from animal-to-human transmission, although the exact details of how they occur are unclear and it has not yet been possible to isolate the same viral strain in animals and in humans. It may come from direct contact with a living animal when hunting or eating bush meat.
One thing we have noticed from our research in the CAR is that the outbreaks tend to be seasonal. This would suggest a link with certain seasonal activities like the harvesting of edible caterpillars, which involves individuals entering the forest, where they would be more exposed to local wildlife.
Even though scientists have been tracking the viral reservoir since the 1970s, it has, as of yet, only rarely been isolated in wild animals. The first instance was in 1985 in the DRC and involved a species known as a Thomas’s rope squirrel (Funisciurus anerythrus), thought to be the reservoir of the virus. The next was that of a sooty mangabey monkey in 1992 (Cercocebus atys) in Côte d’Ivoire. Then, two decades later, the virus was isolated in a Gambian pouched rat and another rodent species (Stochomys longicaudatus), as well as in another rope squirrel (Funisciurus _bayonii) and a shrew (Corcidura litoralis). As things stand, the prime suspects for the viral reservoir are rodents, including squirrels.

The Funisciurus anerythrus squirrel is the suspected reservoir of the monkeypox virus.
cherifikoukomon, CC BY-NC
Interestingly, monkeypox was also found in chimpanzee feces in Taï National Park, Côte d’Ivoire, during an outbreak among primates, which implies the possibility of environmental contamination.
Aside from zoonotic transmission, there’s also human-to-human transmission, which occurs as a result of direct and prolonged contact with infected individuals through exposure to bodily fluids or contaminated materials (e.g. clothing, bedding, or surfaces). Such infections most often take place within the home.
Transmission through inhalation of respiratory droplets has also been considered, but this point is difficult to ascertain. Generally speaking, infections take place within the family home, where there is closer human proximity and modes of contact are numerous and diverse. Africa has also seen some cases of hospital-acquired infection.
In a detailed case description of the 2017 Nigerian outbreak, a large proportion of individuals suffered genital infections (68%), suggesting for the first time that the virus could be transmitted through close skin-to-skin contact during sex. Our data also shows that the rate of such infections is very high among cases recorded in the CAR.
Close, intimate contact during intercourse may be behind the new increased frequency in interhuman transmission of monkeypox, a virus that is usually thought to present low transmissibility. This theory is supported by the fact that – at the time of writing – the “non-African” cases of recent weeks have mainly affected young men who have sex with men or who identify as homosexual. It should be noted, though, that such transmissions could also occur during heterosexual intercourse.
Italian researchers have recently detected significant amount of monkeypox virus in the semen of 3 patients. However, the authors stressed that these findings “cannot be considered definitive evidence of infectivity”. The implications for transmission are not clear either.
TC: Should we be worried about this disease becoming widespread? What can we do to prevent this?
CB: For now, we can’t say for certain what will happen. The problem is that the chain of transmission of these new cases has yet to be identified. As indicated by the daily evolution of the virus, and due to its relatively long incubation period, there is a real risk that new infections could emerge within the coming days and weeks, whether in countries that are already affected or elsewhere.
Numerous cases in Spain and Europa appear to be linked to two festivals, one in Belgium between 4 and 9 May and one that took place in the Canary Islands between 5 and 15 May. The latter was attended by 80,000 people, potentially making it a “super-spreading” event.
In order to prevent the spread of the virus, we need to raise awareness among the communities and individuals concerned, and among doctors, so that we can quickly identify each case and trace their contacts. One difficulty facing doctors is that monkeypox lesions resemble those caused by chickenpox and, when they occur on the genitals, they can be mistaken for symptoms of some STIs (like syphilis and herpes). A monkeypox diagnosis can be confirmed by a PCR test and isolation of the virus, but only a few specialist laboratories are equipped for these types of analysis.
Nevertheless, we can find some reassurance in the fact that monkeypox outbreaks resolve spontaneously and relatively quickly. The longest chain of transmission ever identified was carried over seven generations, meaning that seven humans passed on the disease consecutively before transmission ceased.
It is unclear why spreads simply stop like this. One hypothesis is that, until recently, these outbreaks would occur in small villages within restricted communities where some individuals might have already been immune, so the virus would contaminate only those who had never been in contact with it. But the 2003 epidemic in the United States also ended quickly and without any secondary human-to-human infection.
It remains to be seen where this new outbreak will take us.
TC: Could the smallpox vaccine protect against this virus?
CB: We know that a natural infection of smallpox offers cross-protection against monkeypox. In the 1980s, it was shown that the smallpox vaccine could also provide cross-protection at a rate of around 85%. However, these estimates were made just a few years after the mass-vaccination campaign to eradicate smallpox. It is now believed that its efficacy is closer to around 63% against severe disease.
Moreover, smallpox vaccination was suspended in the 1980s once the disease was wiped out. Today, only a handful of healthcare professionals are vaccinated (in case of bioterrorist threats, while the virus is stored under high surveillance at a number of laboratories) and the first generation of the vaccine is no longer used, due to significant side effects.
At present, if ever necessary, the most suitable vaccine for a roll-out would be the “third-generation” version known as Imvamune (or Imvanex or Jynneos). This is an attenuated vaccine that can be administered to immunocompromised people, unlike older vaccines. It has already been given to healthcare professionals and contact cases in Israel, Singapore, and the UK, and its effectiveness is currently being assessed among healthcare professionals in the DRC.
There are also a number of fourth-generation “subunit” vaccines in development. These do not contain the attenuated virus and instead have only fragments of it. They are also being assessed for their effectiveness.
Vaccines can be administered as either a pre-exposure (i.e. before contact with the virus) or a post-exposure treatment. With regards to the latter, US guidelines recommend that it be given preferably within 4 days and up to 14 days after exposure. On 27 May two French individuals received post-exposure vaccination for the first time after a high-risk contact with confirmed cases.
TC: Could we see other variants of monkeypox emerging? Is the genome of the virus currently circulating in Europe identical to the West African strain?
CB: Monkeypox is a DNA virus, which means that it is less likely to mutate than an RNA virus like SARS-CoV-2.
It is actually quite simple to determine whether we are dealing with a West African or Congo Basin strain. We just need to sequence out short sections of its DNA. But given the large size of the viral genome, it takes time and effort to obtain a complete sequence. We need this complete sequence in order to detect differences in sequences more precisely, which would allow us to identify chains of transmission and find out how cases are linked. However, if our experience with SARS-CoV-2 has taught us anything, it’s that a large-scale global effort can be of great help in moving things along.
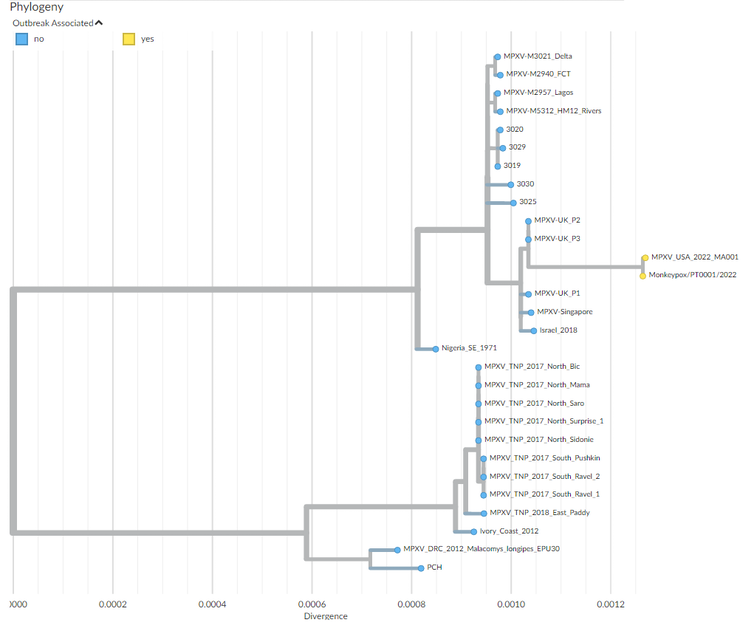
Phylogenetic tree depicting the ‘family’ relationships between the different strains of monkeypox virus responsible for outbreaks.
Nextrain.org
Initial sequencings carried out on samples from a Portuguese and a Belgian patient have shown the genetic proximity of the virus to strains isolated in Nigeria and during the previous out-of-African spread of the virus in 2018, with genomes of the ongoing outbreak being highly similar. This is in favour of a single introduction followed by community spread in Western countries after superspreading events.
More detailed genomic analyses comparing 2022 strains to those of 2018 identified around 40 mutations (fivefold the expected rate of mutations) with a pattern specific of the action of an antiviral enzyme called APOBEC which may reveal the sustained circulation of the virus in a new animal intermediate host, or in humans. This observation, possibly indicating a recent increase in viral circulation in Nigeria, matches the documentation of cases in peri-urban areas of Nigeria like Abuja, together with increased frequencies of overseas exportation of cases.
A recent article hypothesizes that Nigerian synanthropic rodent populations (i.e. undomesticated rodents that live in close association with people and benefits from their surroundings) have increased in recent years as a result of land conversion and high urbanization leading to increased human-rodent contact.
Further sequencing is required to address remaining questions, like genome adaptation towards increased viral transmissibility. But, for the time being, there has been no evidence to suggest this.
In a more exceptional way, what looks like a relapse of the monkeypox disease was reported among one of the 2018 UK patient, with an increased lymphadenopathy, a recurrence of rash and a transient shedding of monkeypox viral DNA following initial complete recovery. This hypothesis requires further study to be exploited.
TC: In 2019, the Institut Pasteur came together with partners in France and the CAR to launch the Afripox project, driven by a goal to increase understanding of the monkeypox virus and its spread. What exactly does the project involve?
CB: Afripox is a cross-disciplinary project that was set up in light of an increasing number of monkeypox outbreaks in the CAR, as reported by Emmanuel Yandoko Nakoune, Director of the Laboratory for Arboviruses, Viral Haemorrhagic Fevers, Emerging Viruses, and Zoonoses at the Institut Pasteur in Bangui, the country’s capital.
In the past few decades, monkeypox outbreaks have been more numerous and frequent in Africa overall, with the disease also expanding into areas where it was not endemic before. Improved medical monitoring and reduced immunity (following the end of smallpox vaccinations in 1980) are likely to have contributed to this figure, but the phenomenon may also reflect a growing viral circulation in a region of the world currently experiencing major ecological disturbances.
Faced with the many uncertainties surrounding the epidemiology of monkeypox, the idea for this project was to rely on the CAR’s existing national medical monitoring system to develop a One Health approach toward the monkeypox virus, encompassing all its aspects in epidemiology, ecology, zoology, anthropology, and virology.
For instance, through our partnership with researchers from the French National Museum of Natural History, we are attempting to identify its animal reservoir. Meanwhile, along with the SESSTIM team in Marseille, we are exploring the disease’s ecology in order to better understand why it spreads more in forest areas, pinpoint how deforestation affects outbreaks, determine whether or not there is a seasonal aspect, and so on.

Emmanuel Nakoune and Camille Besombes investigating an outbreak of monkeypox in Zoméa, Lobaye, CAR.
Jean Marc Zokoé, Author provided
In the near future, the Afripox project also hopes to use on-the-ground PCR diagnostic tests that are currently being developed by the Emergency Biological Response Unit (“Cibu”) team at the Institut Pasteur Paris. For now, suspected case samples are analyzed in Bangui, but these tests would allow for reduced diagnosis time and quicker implementation of the appropriate measures.
Lastly, the epidemiological and anthropological aspects of the virus are being explored by the teams at the Institut Pasteur Paris (namely, the Emerging Diseases Epidemiology Unit and the Emerging Diseases Anthropology and Ecology Unit), in collaboration with local researchers. Their goal is to precisely determine the risk factors of zoonotic or interhuman transmission and ascertain why monkeypox has been on the rise since the 1980s. While it is essential to identify the mechanics of this latest human-to-human epidemic of a relatively new format, it is also vital to understand how monkeypox emerges and circulates in its continent of origin.
When Afripox was launched three years ago, few could have imagined that this disease would one day spread beyond the African continent and across the planet. The current epidemic has highlighted once more the importance of investing in scientific research over the long term, so that we can be better prepared for any and all eventualities.
Translated from the French by Enda Boorman for Fast ForWord







