Methods for Including Adverse Events in Economic Evaluations
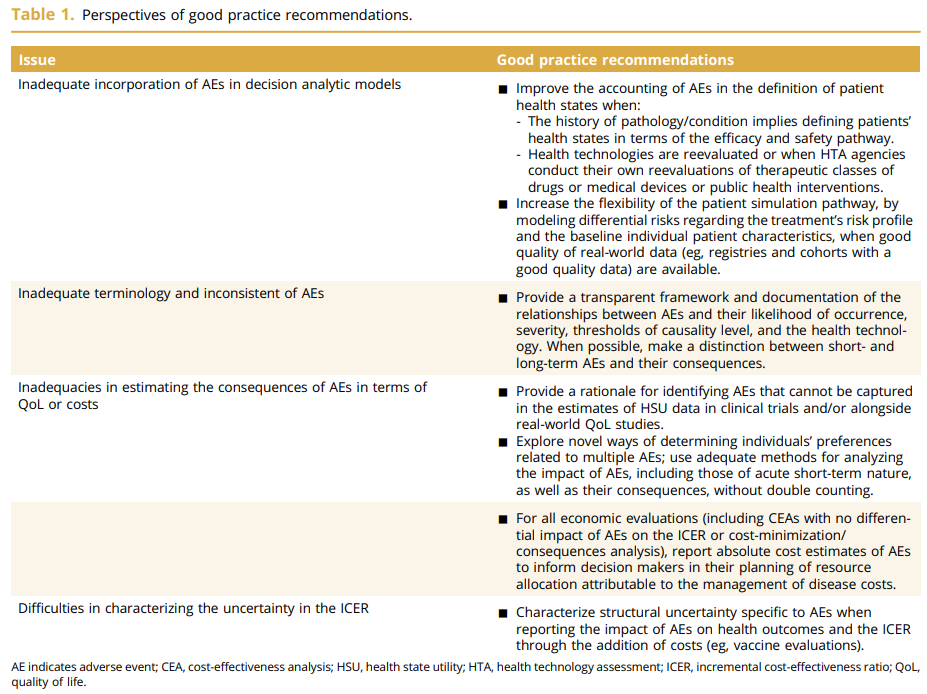
That is the title of a great review paper by Salah Ghabri, Dalia Dawoud and Michael Drummond. They discuss that guidance on the incorporation of adverse events (AEs) into economic models has not been standardized. Nevertheless, this paper provides helpful guidance. Specifically, the paper explores: (1) the incorporation of AEs in economic decision models, (2) the types of AEs to be included, (3) the estimation of their consequences in terms of both costs and QoL, and (4) the exploration of uncertainty related to their impact on the ICER. I summarize each issue in turn.
1. Ways to incorporate AEs into economic models:
Included in analytic model structure. For instance, AEs could be a separate health state. The authors cite a paper by Stevenson et al. (2016) which evaluates the cost-effectiveness of new
rheumatoid arthritis treatments. Their model included patients’ health states defined with moderate-to-good response at 6 months without serious AE or patients without response and, or experiencing a serious AE. This approach is most useful if AEs are serious and relatively common. Included as input parameters. With this approach, AE input parameters are incorporated into each health state. For instance, parameterization with respect to rates of incidence, time to onset, loss of QoL, and costs for each AE would be applied to each relevant health state. This approach is most commonly used in the literature. Often, AEs are modelled from clinical trial data and are only incorporated in the first model cycle or first year; long-term consequences of AEs are often ignored.
2. Terminology and types of AEs to be included
Adverse events are most often referred to as such, but sometimes other terms are used such as: toxicity, side effects, adverse drug effects, drug effects, serious infection or adverse reaction. One key differentiation between AEs are that some are chronic in nature and some are acute.
While AEs are often identified from clinical trials, real-world data can be used. For pharmaceuticals, post-market surveillance of safety is mandatory, but medical devices this is not always the case (depending on the country).
3. Estimatation of AE Impact on QoL and Costs
There has been some methods guidance on how to estimate the impact of AEs on quality of life. This is most often done using utility decrements which subtract a fixed amount of utility from each relevant health state. However, this subtracting of AE QoL impacts should only occur if the utility impact isn’t already captured by the health state QoL. Moreover, to estimate the utility decrement of AEs, the authors write:
For estimating the utility decrement corresponding to an AE, Pullenayegum et al. showed the conditions where methods, such as tobit or censored least absolute deviation models, should be considered with caution. Rather, alternative regression approaches (eg, linear models of mean utility) should be used.
Another challenge is the frequency with which AEs occur. Often, clinical trials report the share of patients who have experienced a given AE, but not how often that AE occurs (nor its duration). On the cost side, most models focus on hospitalization costs for acute and severe events (often defined as grade ≥3 AEs).
4. Uncertainty around impact of AEs on ICER
Because AEs observed during clinical trials cannot be extrapolated to estimate impact on long-run AEs or overall health outcomes, conducting scenario analyses around AEs is rarely done. However, in many cases, long-run AEs may be important. For example:
…[an] economic evaluation related to smoking cessation (Keeney et al.) showed that the exclusion of AEs associated to depression and self-harm affected the determination of the most cost-effective health strategies.
Despite these challenges, the authors do offer some best practices to help researchers address them.
A very interesting article throughout. You can read the full paper here.







