Is TEE the answer for incentivizing the development of new antibiotics?
Antibiotic resistance is an increasing problem. My own research shows that when infections are antibiotic resistance, the risk of complications rises as does the cost to treat the infection. According to the CDC, antimicrobial resistance is a major public health problem:
…killing at least 1.27 million people worldwide and associated with nearly 5 million deaths in 2019. In the U.S., more than 2.8 million antimicrobial-resistant infections occur each year. More than 35,000 people die as a result…
Given the global health burden, one would think pharmaceutical firms would rush to invest in antibiotics. However this is not the case. An article in Nature summarizes the issue:
A 2017 estimate puts the cost of developing an antibiotic at around US$1.5 billion1. Meanwhile, industry analysts estimate that the average revenue generated from an antibiotic’s sale is roughly $46 million per year. “That’s tiny and nowhere near the amount needed to justify the investment,” says Kasim Kutay, chief executive of Novo Holdings, an investment firm
Revenue from antibiotics is low because there are many low-cost, generally effective antibiotics that get used for most infections; new antibiotics are used only for the limited (but growing) number of antibiotic resistance. Moreover, stewardship concerns mean that physicians should try to not use newer antibiotics; increasing use of new antibiotics increases the risk of resistance to these drugs. An article by Klug et al. (2020) notes that this is the ‘fire extinguisher problem’ which is an object that is important to have, but not to use. Venture capitalists are also generally lack interest in investing in antibiotic development.
One potential solution to this issue is a Transferable Exclusivity Extension (TEE) system. EFPIA commissioned reports on this topic in 2019 and in 2022. In this system
…the manufacturer of a new antimicrobial which meets certain criteria would receive a voucher (‘a TEE’) upon European regulatory approval of that antimicrobial. This voucher can be used by the recipient to extend the marketing exclusivity of one of its products for a period of time, or sold to another company which can then use it to extend the marketing exclusivity of one of its own products. TEE has been put forward as an incentive that would have sufficient power to incentivise antimicrobial research and development. It is within current European Union (EU) competencies and would provide pull incentive funding in a stable manner, not dependent on appropriations from Member States (MS) and with no up-front cost, but there are also concerns about its use in Europe, particularly regarding the costs of the TEE.
The 2022 report aims to quantify the benefits and costs of the TEE system. The benefits of TEE are hard to estimate. While the benefit of having an additional antibiotic may be estimable, the likelihood many of our current antibiotics become ineffective is hard to estimate. It is somewhat of a black swan event, one that is relatively unlikely in any given year, but may be more likely over a longer time horizon and one that would be disastrous for society. Further, antibiotic resistance would impact not only individuals with the disease but also broader sources of value including:
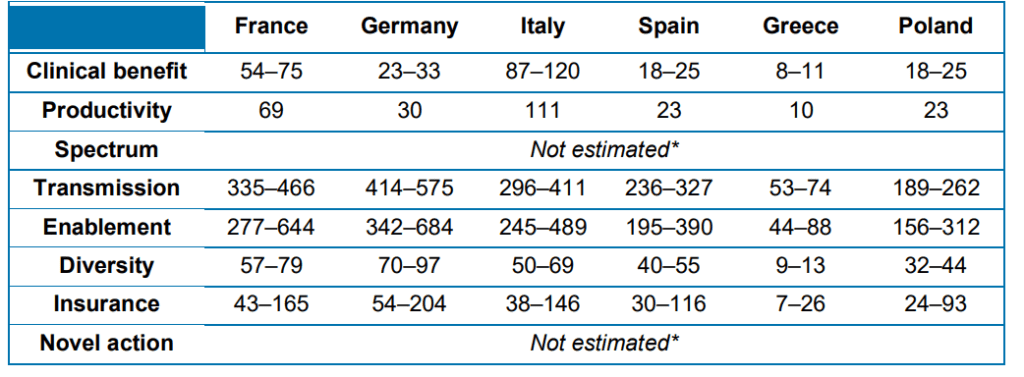
Spectrum value. Benefits of replacing broad-spectrum with narrow-spectrum antibiotics that target specific pathogens to prevent ‘collateral damage’ to the microbiome and reduce AMR build-up. Transmission value. Benefits of avoiding the spread of infection to other individuals in the population. (See Morton et al. 2019) Enablement value. Benefits of enabling surgical and medical procedures to take place. (see Morton et al. 2019) Diversity value. Benefits of having a range of treatments available to reduce selection pressure and preserve the efficacy of existing antibiotics (see Teillant et al. 2015) Insurance value. Benefits of having treatments available in case of sudden, or major, increase in incidence of a certain bacterial infection. (see Megiddo et al. 2018)Novel action value. Benefits of having a new mechanism of action (MOA) that helps prevent cross-resistance developing among classes of antibiotics and paves the way for ‘follow-on’ products with the same MOAProductivity. Impact on employee efficiency and overall economic activity. (see Codecasa et al. 2015)
Interestingly, the majority of the benefits of TEE are not direct clinical value to infected patients but rather transmission value, enablement value and insurance value. The table below provides these estimates.
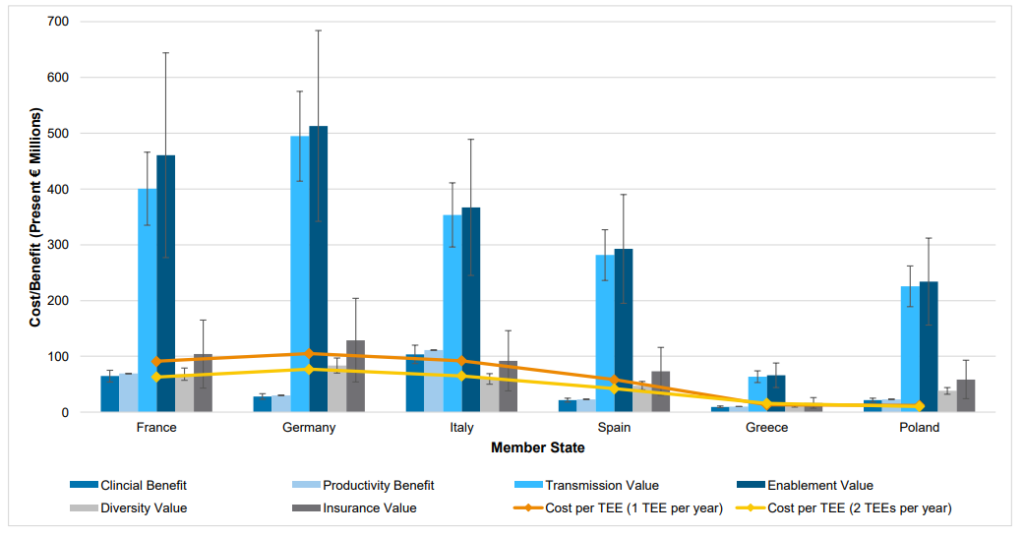
On the other hand, the cost of implementing a TEE are largely due to two factors: (i) higher drug prices for the drug to which the TEE voucher is eventually applied, and (ii) additional administrative costs. Unsurprisingly, the 2022 report. estimates that about 98% of the additional cost is from higher drug prices and only 2% is from higher administrative costs. The authors find that a TEE voucher for 1 antibiotic per year would cost European countries in the hundreds of millions of euros. The cost for a 12 month TEE voucher to different European nations is €121m for Germany, €105m for France and Italy each, €66m for Spain, €15m for Greece, and €14m for Poland.
On net the report finds that TEE benefits more than offset potential cost. Details of the specific benefits and costs are listed in the figure below.
Key Papers
Morton, A., Colson, A., Leporowski, A., Trett, A., Bhatti, T. & Laxminarayan, R. (2019) How Should the Value Attributes of Novel Antibiotics Be Considered in Reimbursement Decision Making? MDM Policy & Practice. 4(2) https://doi.org/10.1177/2381468319892237 Teillant, A., Gandra, S., Barter, D., Morgan, D. & Laxminarayan, R. (2015) Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. The Lancet, 15(12): 1529–1437. https://doi.org/10.1016/S1473-3099(15)00270-4Rothery, C., Woods, B., Schmitt, L., Claxton, K., Palmer, S. & Sculpher, M. (2018) Framework for value assessment of new antimicrobials: implications of alternative funding arrangements for NICE Appraisal. EEPRU. Available at: https://pure.york.ac.uk/portal/en/publications/framework-for-value-assessment-of-new-antimicrobials(f0bc0ec9-9236-4cc7- 9498-0002bd31f429).html Megiddo, I., Drabik, D., Bedford, T., Morton, A., Wesseler, J. & Laxminarayan, R. (2018) Investing in antibiotics to alleviate future catastrophic outcomes: What is the value of having an effective antibiotic to mitigate pandemic influenza? Wiley Health Economics. 28(4): 556–571. https://doi.org/10.1002/hec.3867Codecasa, L., Toumi, M., D’Ausilio, A. et al. (2017) Cost-effectiveness of bedaquiline in MDR and XDR tuberculosis in Italy. J Mark Access Health Policy. 5(1): 1283105. https://doi.org/10.1080/20016689.2017.1283105







