ICER’s proposed changes to their value framework
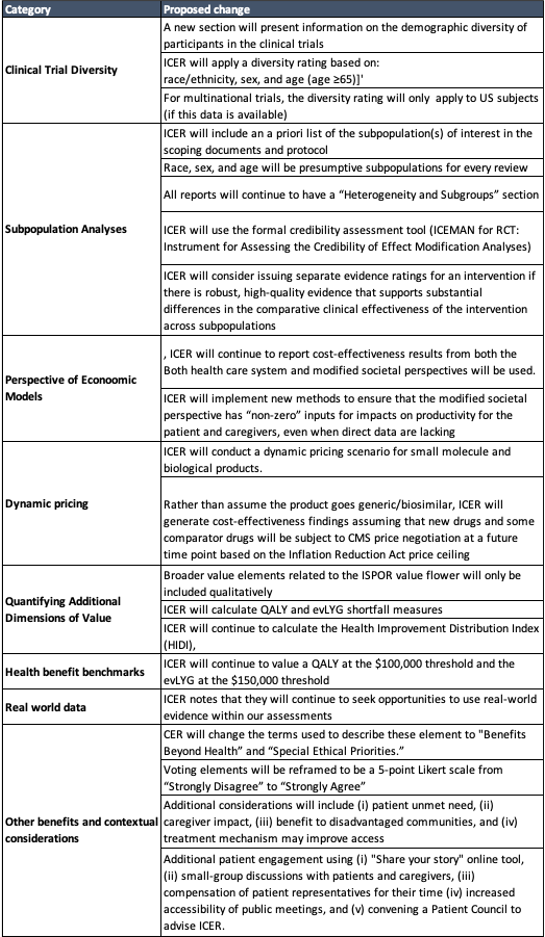
This week ICER released a number of potential changes to their value framework. A summary of these are listed in the table below.
Clinical Trial Diversity
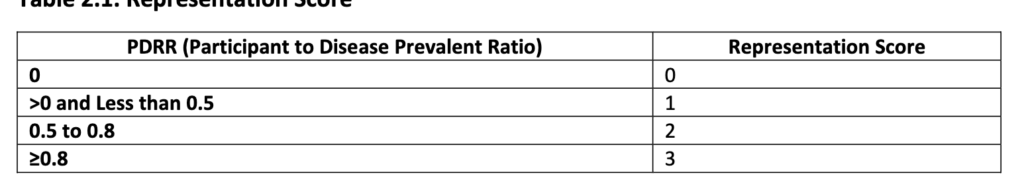
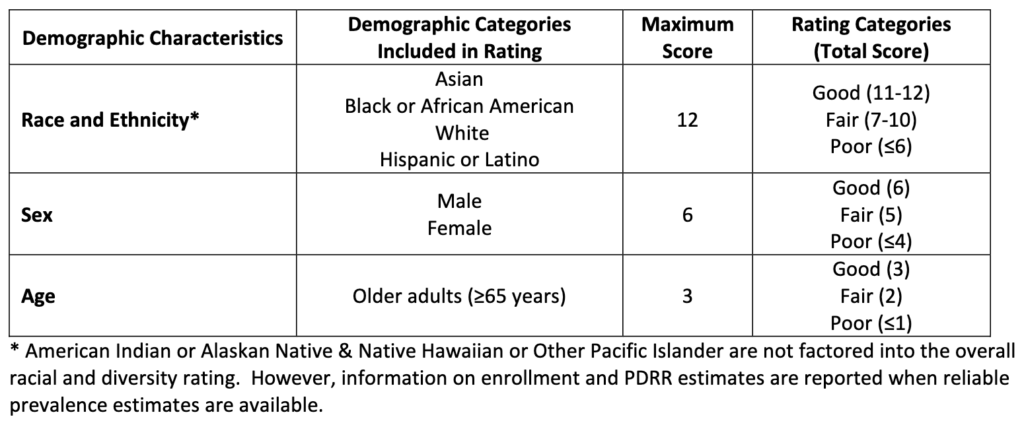
The clinical trial diversity measure is calculated based on a ratio people in the trial compared to the population of patients with the disease in the US. The categories over which this is measured is race, age and sex. The tables below describe the calculation. However, it does not appear that the Clinical Trial Diversity measure would impact the value-based price.
 https://icer.org/wp-content/uploads/2023/06/Proposed-VAF-Changes-For-Public-Comment_For-Publication_06052023.pdf
https://icer.org/wp-content/uploads/2023/06/Proposed-VAF-Changes-For-Public-Comment_For-Publication_06052023.pdf
 https://icer.org/wp-content/uploads/2023/06/Proposed-VAF-Changes-For-Public-Comment_For-Publication_06052023.pdf
https://icer.org/wp-content/uploads/2023/06/Proposed-VAF-Changes-For-Public-Comment_For-Publication_06052023.pdf
Heterogeneity and Subgroups
The ICEMAN for RCT approach aims to answer the following questions to evaluate the overall credibility of a subgroup claim.
Was the direction of the effect modification correctly hypothesized a priori?Was the effect modification supported by prior evidence?Does a test for interaction suggest that chance is an unlikely explanation of the apparent
effect modification?Did the authors test only a small number of effect modifiers or consider the number in their
statistical analysis?If the effect modifier is a continuous variable, were arbitrary cut points avoided
Productivity impact
In cases where there are not strong evidence of how treatments can improve productivity, ICER will use an approach that links patient utilities to productivity. ICER writes:
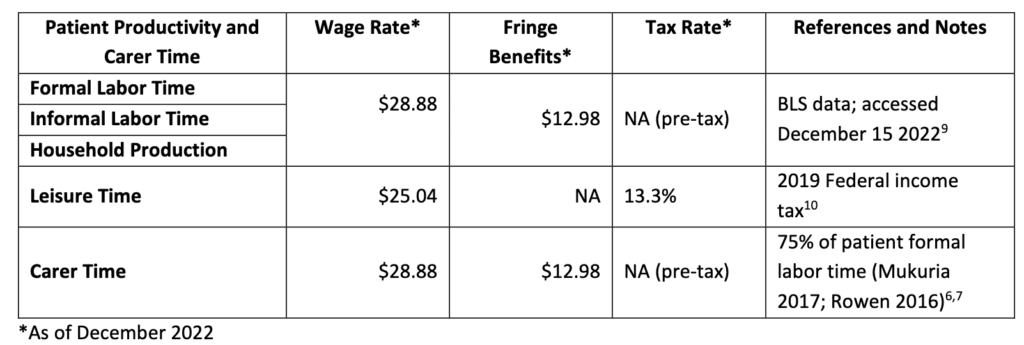
To inform estimates for the indirect approach, ICER will use the published relationship between patient utility scores and patient time use data5 to derive the anticipated impacts of the treatment on time spent in each activity due to the disease and its management for the patient. The indirect approach values productivity time spent in a given health state, which is in contrast to the most typical approach of valuing productivity time lost, creating an opportunity to capture productivity time gained during periods of life extension. In these circumstances, and in line with the published literature[Jiao and Basu 2023], ICER will include patient productivity time gained and patient consumption costs during periods of life extension. Since no parallel relationship between patient utility scores and carer time use data exists for the US setting, ICER will assume that carer time spent is proportional to 75% of patient formal labor time. This estimate is based on the modeled relationship between carer time required[Rowen et al. 2016] and patient time lost[Mukuria et al. 2017] according to patient utility scores in the United Kingdom setting.
Time impact will be valued as follow:
Dynamic Pricing
ICER now will incorporate dynamic pricing as follow:
Net prices will not increase above inflationSmall molecule prices are assumed to fall by 75% in year 9 after launchBiologic treatment prices are assumed to fall by 65% in year 13 after launchBoth the treatment and comparator interventions will be subject to this dynamic pricing assumptionsThis approach, however, would not allow for changes in the cost-effectiveness findings for one-time cell or gene therapies
Willingness to pay per QALY
ICER writes:
The starting point for ICER’s HBPB range uses the health care system perspective threshold-based prices from the highest and lowest annualized price within the $100,000 to $150,000 per QALY and per evLYG. The most common ICER HBPB range includes the treatment’s price that meets the $100,000 per QALY gained on the low end and meets the $150,000 per evLYG on the high end of our range…ICER will continue to provide threshold-based prices from $50,000 to $200,000 per QALY and per evLYG within our reports.
Single short-term (SST) therapies
When reviewing SST therapies, ICER will use a:
A 50/50 shared savings model in which 50% of the lifetime health system cost offsets from a new treatment are “assigned” to the health system instead of being assigned entirely to the new treatment; andA cost-offset cap model in which the health system cost offsets generated by a new treatment are capped at $150,000 per year but are otherwise assigned entirely to the new treatment.
Topic Selection
ICER notes that health equity considerations will now play a role in their topic selection.







