How are drugs developed and financed? An overview
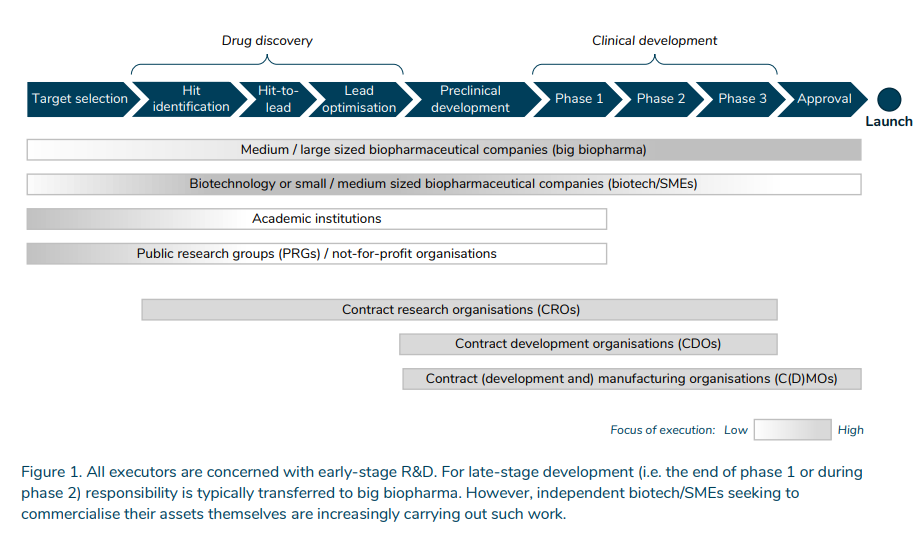
I just came across a nice overview article titled The financial ecosystem of pharmaceutical R&D. The white paper was developed for the Netherlands government and provides an overview of the drug development process and how (and by whom) it is financed. The white paper answers the following questions:
What do different players do within the drug development ecosystem?
Academic institutions and public research groups (PRGs) / not-for-profit organisations 1 are principally concerned with target selection, i.e. identifying disease targets, although they may also play a role in later phases. Biotechnology companies (biotech) or small/medium-sized biopharmaceutical companies (SMEs) are most active in drug discovery, preclinical development and early-stage clinical development. Drug discovery involves finding and optimising a drug candidate that interacts with the disease target. In the preclinical development phase, the safety and efficacy profiles of the drug candidate are tested in animal models and subsequently in human trials in clinical-development phases. Medium/large-sized biopharmaceutical companies (big biopharma) are active throughout the whole value chain. They are the critical late-stage clinical development executors. The responsibility is typically transferred to big biopharma from the end of phase 1 or during phase 2. However, biotech/SMEs seeking to commercialise their assets themselves are increasingly carrying out such work.
https://www.sirm.nl/docs/The-financial-ecosystem-of-pharmaceutical-RD_2022-06-21-193729_afll.pdf
What are typical archetypes of how different stakeholders interact in the drug development process?
The paper also lists 7 different archetypes for drug development.
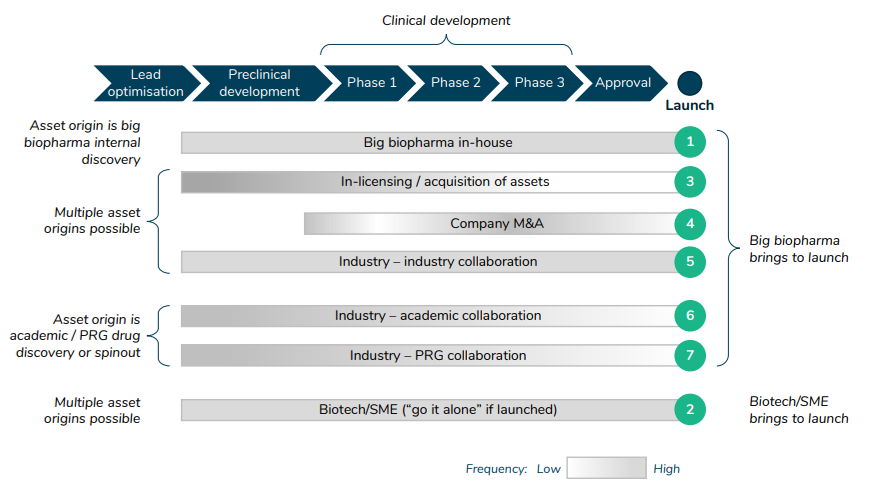 https://www.sirm.nl/docs/The-financial-ecosystem-of-pharmaceutical-RD_2022-06-21-193729_afll.pdf
https://www.sirm.nl/docs/The-financial-ecosystem-of-pharmaceutical-RD_2022-06-21-193729_afll.pdf
Who finances drug development?
About two-thirds comes from private sector life sciences companies. However, we see that between 2011-2019, private sector funding has grown. VC funding has grown by 14.2% per year, biopharma by 4.1%, but public sector and not-for-profit only grew by 1.1% and 0.8% respectively.
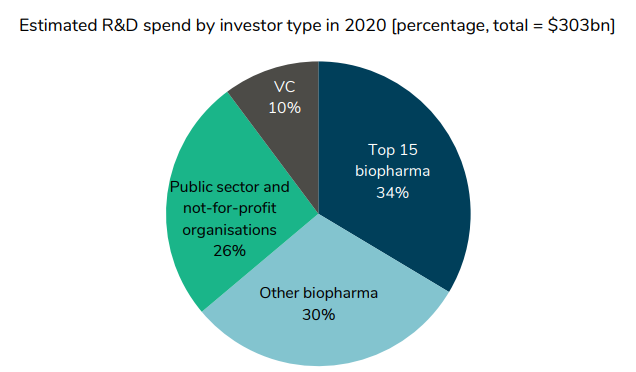 https://www.sirm.nl/docs/The-financial-ecosystem-of-pharmaceutical-RD_2022-06-21-193729_afll.pdf
https://www.sirm.nl/docs/The-financial-ecosystem-of-pharmaceutical-RD_2022-06-21-193729_afll.pdf
A case study of how non-profits and biopharma funding interact is shown by the recent development of numerous cystic fibrosis drugs.
Since only a few therapies were available to treat the symptoms of cystic fibrosis (CF) in the late 1990s, the Cystic Fibrosis Foundation (CFF) looked to support the development of disease-modifying therapies. CFF wanted to make strategic investments in pharma companies aimed explicitly at cystic-fibrosis-therapy development. In 2000, CFF partnered with Aurora Biosciences to identify disease-modifying molecules. Vertex Pharma acquired Aurora Biosciences in 2001 but did not invest heavily in this CF franchise due to a heavy strategic focus on virology. When Kalydeco entered phase 1 trials in 2006, CFF funded an additional $37m. The successful results of this phase encouraged Vertex to invest in building more R&D and commercialisation capabilities for the CF franchise. Moreover, CFF funded an additional $75m after phase 2 trials began. After approval in 2012, Kalydeco became commercially successful. CFF benefited by selling their royalty rights for Kalydeco in a $3.3bn deal they reinvested in CF research
How much does it cost to develop a drug?
It is expensive:
Whether successfully launched or not, an executing company’s out-of-pocket R&D costs for one compound are an estimated $280–$380m. If including the R&D costs of drugs that fail, however, the estimated out-of-pocket costs to the system for developing one approved drug increase considerably to $1.2– $1.7bn (§2.3.2). Adding the cost of capital, the total R&D cost to the system adds up to an estimated $2.4–$3.2bn per single approved drug
More than just the expense, is the uncertainty. Government, non-profit and venture capital helps to bridge the “translation gap” between early stage basic scientific research into target selection and drug discovery and into pre-clinical and clinical trials.
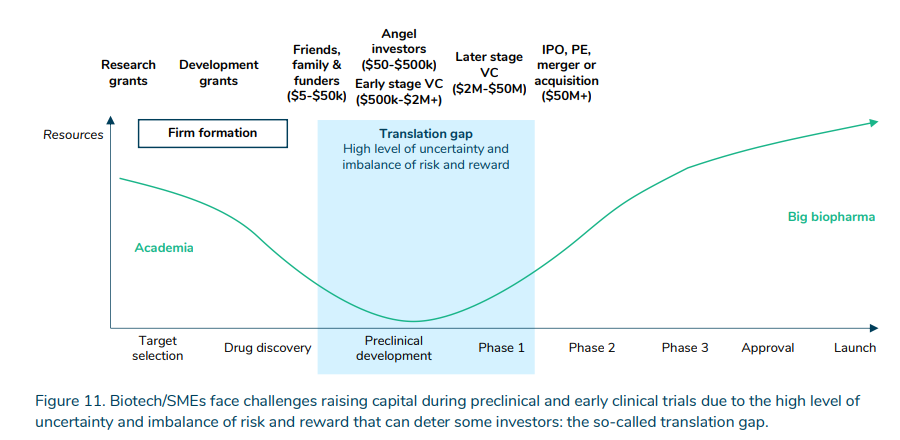 https://www.sirm.nl/docs/The-financial-ecosystem-of-pharmaceutical-RD_2022-06-21-193729_afll.pdf
https://www.sirm.nl/docs/The-financial-ecosystem-of-pharmaceutical-RD_2022-06-21-193729_afll.pdf
How responsive is R&D investment to drug revenues and/or market size?
A key figure is how responsive drug development is to expected revenue. This elasticity ranges from 0.3 to >2.0, but the figure below focuses on newer studies where the range is 0.3 to 1.0.
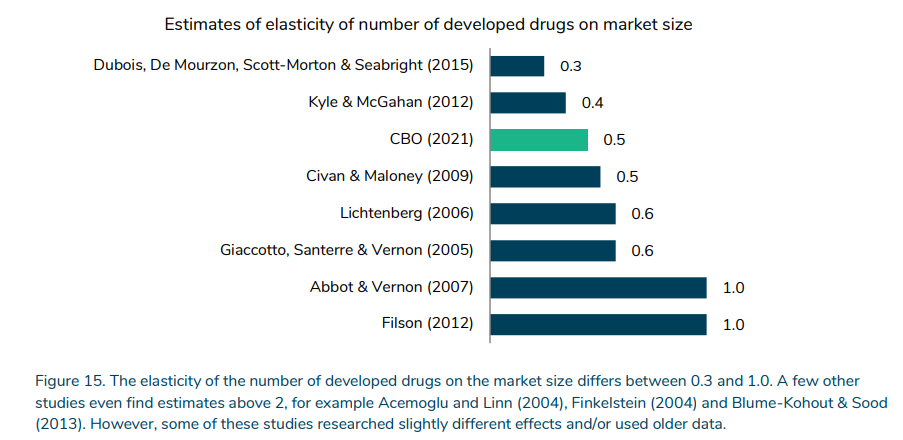 https://www.sirm.nl/docs/The-financial-ecosystem-of-pharmaceutical-RD_2022-06-21-193729_afll.pdf
https://www.sirm.nl/docs/The-financial-ecosystem-of-pharmaceutical-RD_2022-06-21-193729_afll.pdf
The full report can be read here.







