COVID-19 self-testing: it’s time developing countries took the leap
Laboratory testing plays a critical role for diagnosis of COVID-19. It’s the cornerstone of the global public health response, informing control measures and preventing transmission of SARS-CoV-2.
The basis of infectious disease testing depends on the pathophysiology of the infection and the disease that it causes, the clinical course as well as the host immune response.
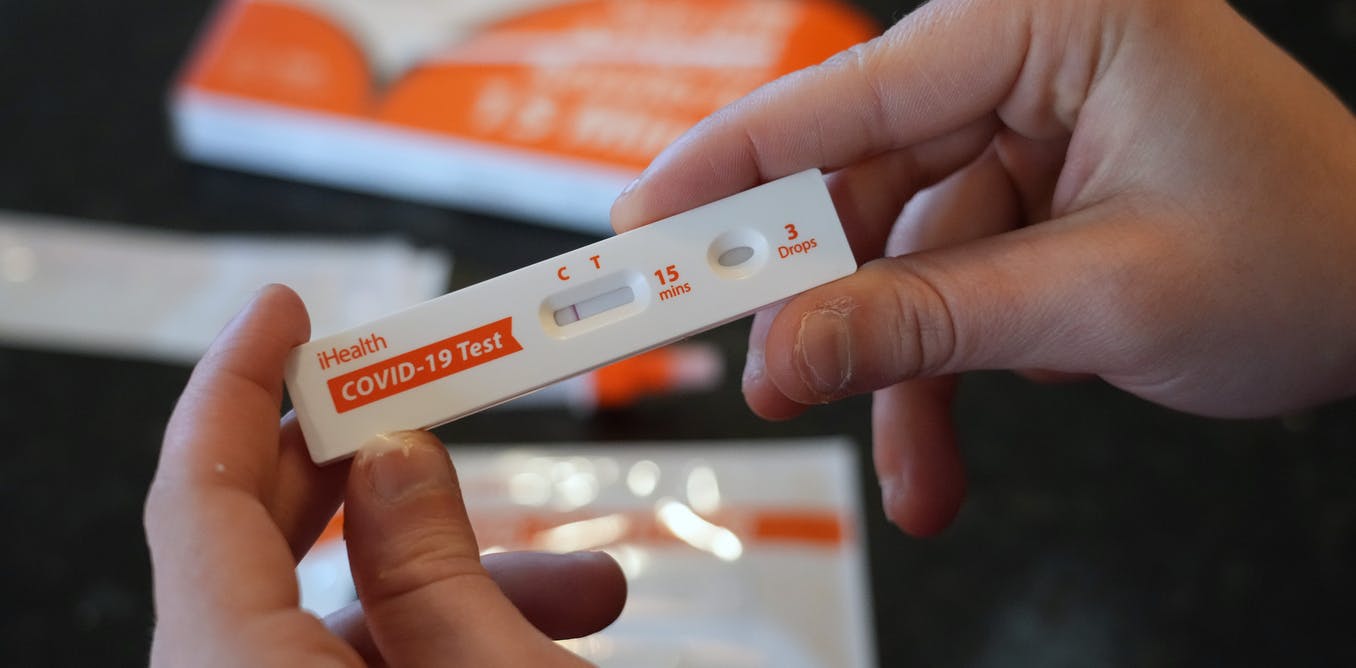
There are two types of tests for acute SARS-CoV-2 infection whether it is symptomatic or asymptomatic: COVID-19 PCR and antigen tests also called “lateral flow tests”. Both detect viral components, but differ in design and complexity.
COVID-19 antigen tests are a game-changer, even at this stage of the pandemic when the world is no longer hoping to achieve containment of SARS-CoV-2 but rather looking at mitigating strategies to prevent infection in those who face the biggest threat from the virus.
Antigen tests are cheap and quick. They can help identify infected individuals and interrupt viral transmission by quarantining infected individuals immediately. In South Africa the proportion of COVID-19 antigen tests compared to PCR remains low. In week 3 of January 191,510 COVID-19 tests were done. Only 22.8% of these were antigen tests. None of these tests were done by individuals – this isn’t allowed under the country’s regulations.
It is difficult to discern why countries in Africa have not taken up COVID-19 antigen testing. Access and price don’t seem to be the issue. The Africa Centres for Disease Control has even published clear guidelines on how to use them. But there is a lingering mistrust in test quality that has limited their use.
It is true that there are concerns about self-testing. But, in my view, the benefits are substantial. Individuals are able to access cheaper and faster tests. This, in turn, helps limit exposure to those at risk of infection. Public health specialists in developing countries are putting pressure on authorities for greater use of antigen tests – particularly for self-testing to be allowed.
The two tests
COVID-19 PCR tests amplify viral gene targets and are very sensitive. Over 1000 commercial ones are now available. They are able to detect viral components slightly before symptoms start in infected individuals and many weeks after infection – even when there is no longer any risk of transmissibility.
The COVID-19 PCR tests have their drawbacks. For example, measures have been put in place – such as hospital admissions and travel bans – on the basis of positive results even though this hasn’t been strictly necessary.
COVID-19 PCR test performance depends on several factors. These include design, the clinical characteristics of the patient, timing of the sample, sample type and transport conditions and laboratory techniques. They are also expensive, technically difficult, require specialised staff and reagents, specimen transport and processing.
Read more:
Antibody tests aren’t a COVID-19 panacea. But they’re a useful additional tool
For their part, COVID-19 antigen tests are immunoassay rapid diagnostic tests that have different viral antigenic targets and designs.
Rapid diagnostic tests are used extensively for the diagnosis of other respiratory infectious diseases like influenza in clinics, community settings and home-based self-testing. Most use a nitrocellulose membrane embedded in a plastic cassette which contains two lines. The one is a control line to inform that the test is working and the second is a detection line to indicate the presence of the virus. The reaction is based on coloured labels (nanoparticles) that change colour when they encounter the target.
COVID-19 antigen tests perform better when there is a high viral load and in patients that are symptomatic. They are usually positive from 5 and up to 12 days after onset of symptoms. They correlate with competent viral replication and thus transmission potential.
Self-testing
Self-testing with COVID-19 antigen tests was first authorised by the US Food and Drug Administration in November 2020 to allow symptomatic people and those that had COVID-19 contacts to test themselves.
By 2021 antigen tests and self testing was firmly entrenched in most developed countries. Their widespread use for self-testing has sparked a range of debates. They hinge on three issues:
Is the test sensitive enough?
A test’s sensitivity is its ability to identify the infection if it is present and also not to miss infections. Sensitivity depends on the test design which in turn impacts its level of detection. Antigen tests differ in sensitivities. Some are excellent compared to PCR tests.
Is the sample adequate?
Simplifying the sample type to include saliva – which is an easier and more standardised sample to access – has increasingly been the direction of most COVID-19 antigen tests.
Is the test result easy to read and interpret?
Reading a rapid COVID-19 antigen test visually may sometimes be difficult. Sometimes faint lines appear in the detection window and it is difficult to work out if the test is positive or negative.
Interpretation is also critical. This is particularly true with negative results. These may not definitively exclude infection. A retest may then be necessary.
In addition, interpreting the result correctly is complex even if clear instructions are provided.
In a large US study on self-testing showed that 1 in 3 individuals misinterpreted their COVID-19 antigen result. The study found that clarity of the information given to the patients doing the self-testing via a variety of different formats was extremely important to a good test outcome.
Individuals in the study drew false reassurance from a negative results.
A recent study also showed that trained laboratory personnel do the tests better than individuals.
What next?
It’s clear that there are a number of caveats to self-testing with antigen tests.
However, on balance, I still believe that they should be allowed more widely in countries like South Africa.
One reason is that the failure to authorise the tests is driving a thriving black market in them. Many are of unknown quality.
Secondly, if used widely they could help control transmission risks as more people would know their status.
However, governments need to issue national guidelines and provide appropriate instructions.







