Challenge in HTA for gene therapies
Last month, the Office of Health Economics published a report titled “Health Technology Assessment of Gene Therapies: Are Our Methods Fit for Purpose?” I summarize some of the key challenges and solutions below.
Challenge #1: Initial assessment of clinical effectiveness. Since gene therapies often target rare disease, the sample size from clinical trials is often small and many trials may rely on single-arm trials. Further, if gene therapy requires a surgeon to administer, real-world effectiveness may vary based on a surgeon’s skill level. Further, less is known about rare diseases in terms of their current treatment pathways and estimates of patient quality of life. I discuss many of the challenge in HTA assessment for rare disease in a recent white paper “Challenges in Preserving Access to Orphan Drugs Under an HTA Framework“Challenge #2: Uncertainty over long-term outcomes. Gene therapies promise long-term gains in health through not only addressing symptoms but fixing the underlying physiology through gene modification. However, clinical trials are typically short-term and often rely on surrogate outcomes rather than the key outcomes of interest that patients care about. Further, gene therapies promise a ‘cure’ but it is unclear note only what proportion of patients will need re-treatment, but whether gene therapies would make future treatments more or less effective. Further, if standard discount rates are applied, long-term health benefits may be too heavily discounted. Challenge #3: Link to value. Gene therapies are currently expensive. Manufacturing gene therapies is much more complex and costly than manufacturing small molecules. Further, many gene therapies are targeted to rare diseases. In fact, 72% of rare diseases have genetic origins. For gene therapies for rare disease, reducing the price of gene therapies may lead to limited investment in rare disease by life science companies and condemning patients with rare disease to limited treatments. Keeping prices high for gene therapies mean that payers may not be getting good value for money as defined by standard cost-effectiveness analyses. Even if treatments were linked to value, HTA often do not incorporate broader value elements such as caregiver burden or more novel value elements such as the value of hope, disease severity, real option value and scientific spillovers among others. New approaches beyond standard CEA–such as generalized risk adjusted cost effectiveness (GRACE) or multi-criteria decision analysis (MCDA)–may prove useful for gene therapies. Challenge #4. Assessment of costs. If gene therapies are paid for with a one-time fee, it is problematic not only through putting pressure on payer budget, but also high costs would be irrecoverable if a treatment were ineffective (as compared to traditional therapies where the treatment could be stopped if ineffective). Additionally, for private insurers, payers may fund a gene therapy only to have the individual switch health plans and thus the entity paying for the gene therapy may not reap its rewards in terms of cost offsets.
Some other challenges include the disability paradox.
Evidence of a disability paradox has been reported in several therapeutic areas targeted by gene therapies. Also known as disease-state adaptation, the disability paradox is where patients of chronic lifelong diseases rate their quality of life as good or excellent despite being perceived to have a lower quality of life by others without disabilities (Albrecht and Devlieger, 1999)
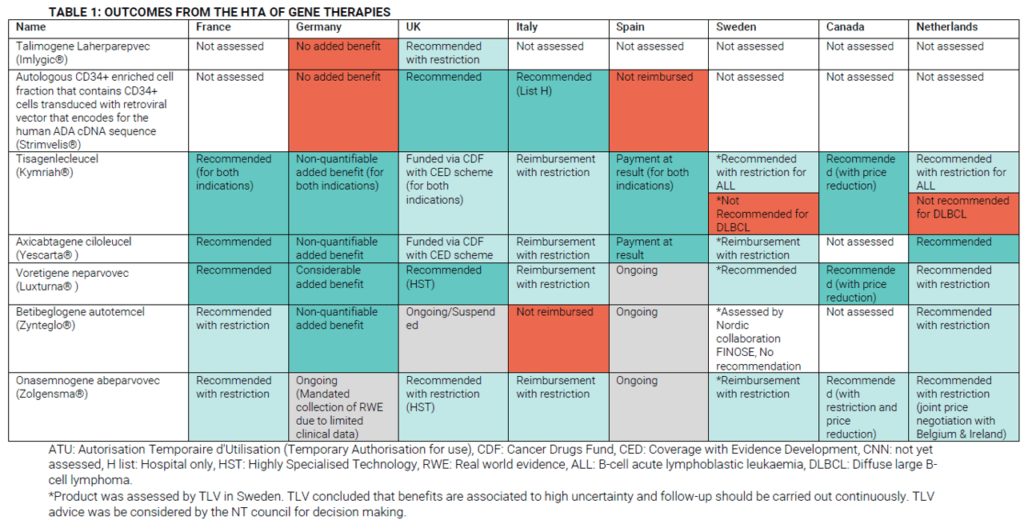
HTA decisions have been made for a number of gene therapies. OHE summarizes some of these decisions across Europe.
Access considerations are not a trivial issue.
The price obtained for a treatment is an important commercial consideration for manufacturers. Pricing constraints in some health systems may lead to inequity of access, with some manufacturers choosing not to pursue reimbursement in some countries as a result. a. This has occurred in practice with Bluebird’s withdrawal of Betibeglogene autotemcel (Zynteglo®) from European markets (Pagliarulo, 2021).
OHE recommendations include:
Take the long view. OHE recommends measuring health outcomes over a lifetime perspective to capture the full long-term value of gene therapies. Sensitivity analyses will be vital as the long-run extrapolation of potential benefits likely will have a significant impact on estimates of treatment value. Think broadly. OHE recommends considering additional value elements as part of the HTA decision process. While there is general consensus that disease severity should impact treatment valuations, there is not much consensus on which other value elements should be included and if so how they should be weighted, despite academics calls for their inclusion. Develop transparent standards for the inclusion of RWE and surrogate endpoints in HTA. While RCTs are of course preferred, OHE wised recommends that “HTA bodies need to demonstrate flexibility in accepting alternative forms of evidence where appropriate.” Further, given the potential long-run impact of the treatments, use of surrogate endpoints may be entirely reasonable for many diseases. Consider outcomes-based arrangement. Due to the high up-front cost of gene therapies and uncertainty over long-term outcomes at drug launch, outcomes-based arrangements or other value-based arrangements may be useful to address uncertainty in long term outcomes while enabling patient access. One simple approach would be amortization of payments. Value of information analysis can be used to inform the arrangements of these agreements (see Drummond et al. 2019). Outcomes-based pricing is increasingly being used to facilitate access to gene therapies, particularly in Germany, Spain, and Italy, but that there is a large degree of variability in HTA methodologies across countries (Jørgensen, Hanna and Kefalas, 2020).International collaboration. OHE recommends expanding data collection through registries and international collaboration. More data collection and international collaboration is always good in theory, but there are costs associated with this and the logistics of implementing cross-border collaboration may be challenging. Nevertheless, it is a wise recommendation and there have been some successes. The French National Rare Disease Plans (PNMR) have created a national database of Rare Diseases (BNDMR). Enable early multi-stakeholder dialogue to align on feasible and appropriate evidence packages. Of particular interest would be establishing early dialogue between manufacturers and HTA bodies as needs for regulatory and HTA approvals may vary; EUnetHTA could help facilitate this collaboration. For instance, “One of the key obstacles to patient access was most HTA bodies’ reluctance to accept single-arm trial evidence, despite traditional RCTs being seen as unethical by some in this circumstance.” Additionally, being patient-centered is important and getting patient and caregiver perspectives on the evidence to be included in HTA evidence packages is important. Disease-specific patient reported outcome (PRO) instruments are difficult to develop for rare diseases so additional weight may be given to patient surveys or patient input during appraisal committee meetings.







